14.5 Storage cells
Electrochemical cells can be used as a commercial source of electrical energy.
Cells can be described as primary, secondary or fuel cells.
Primary cells are non-rechargeable.
Secondary cells are rechargeable.
Battery = collection of cells!
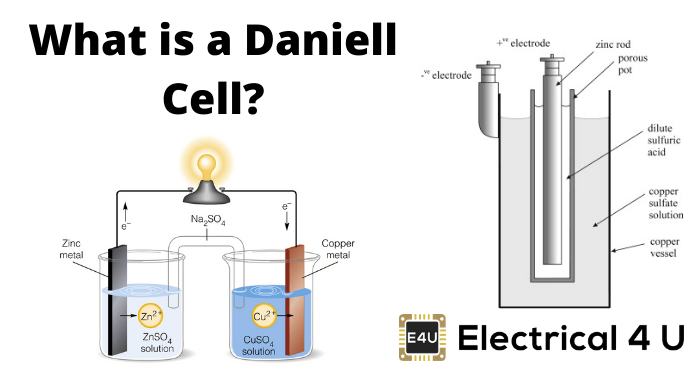
A Daniell Cell is an example of a storage cell that uses zinc and copper electrodes.
Cells can be described as primary, secondary or fuel cells.
Primary cells are non-rechargeable.
Secondary cells are rechargeable.
Battery = collection of cells!
A Daniell Cell is an example of a storage cell that uses zinc and copper electrodes.

3