14.6 Fuel cells
Fuel cells produce voltage by using hydrogen and oxygen as raw materials, and releases water as a waste product.
Fuel cells can be alkaline or acidic, depending on the ion that moves across the electrolyte.
Acidic fuel cells
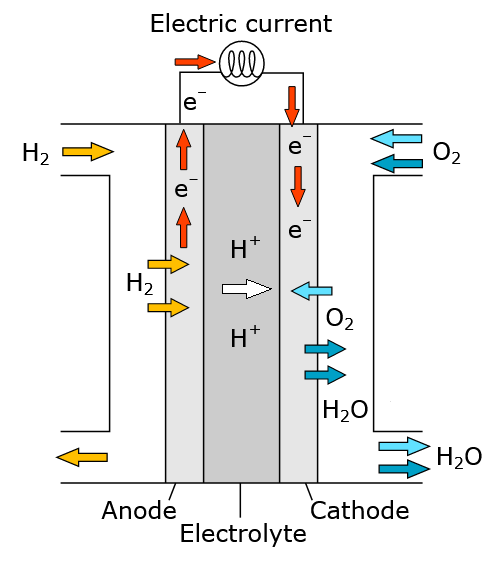
Half equations:
\(2H^+_{(aq)} + 2e^- ⇌ H_{2 (g)} \qquad E^θ = 0.00 V \)
\(\frac{1}{2}O_{2 (g)} + 2H^+_{(aq)} + 2e^- ⇌ H_2O_{(l)} \qquad E^θ = +1.23 V \)
Overall:
\(H_{2 (g)} + \frac{1}{2}O_{2 (g)} ⟶ H_2O_{(l)} \qquad E^θ_{cell} = +1.23 V \)
Alkaline fuel cells
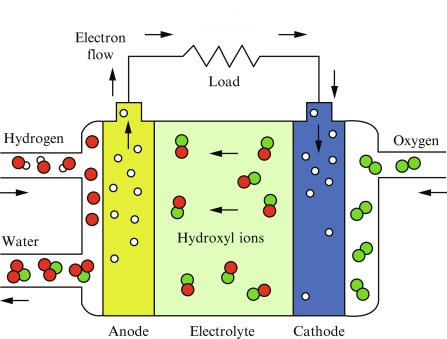
Half equations:
\(2H_2O_{(l)} + 2e^- ⇌ H_{2 (g)} + 2OH^-_{(aq)} \qquad E^θ = -0.83 V \)
\(\frac{1}{2}O_{2 (g)} + H_2O_{(l)} + 2e^- ⇌ 2OH^-_{(aq)} \qquad E^θ = +0.40 V \)
Overall:
\(H_{2 (g)} + \frac{1}{2}O_{2 (g)} ⟶ H_2O_{(l)} \qquad E^θ_{cell} = +1.23 V \)
Non-hydrogen fuels
Methanol and other hydrogen-rich fuels can be used in fuel cells.
Fuel cells can be alkaline or acidic, depending on the ion that moves across the electrolyte.
Acidic fuel cells

Half equations:
\(2H^+_{(aq)} + 2e^- ⇌ H_{2 (g)} \qquad E^θ = 0.00 V \)
\(\frac{1}{2}O_{2 (g)} + 2H^+_{(aq)} + 2e^- ⇌ H_2O_{(l)} \qquad E^θ = +1.23 V \)
Overall:
\(H_{2 (g)} + \frac{1}{2}O_{2 (g)} ⟶ H_2O_{(l)} \qquad E^θ_{cell} = +1.23 V \)
Alkaline fuel cells

Half equations:
\(2H_2O_{(l)} + 2e^- ⇌ H_{2 (g)} + 2OH^-_{(aq)} \qquad E^θ = -0.83 V \)
\(\frac{1}{2}O_{2 (g)} + H_2O_{(l)} + 2e^- ⇌ 2OH^-_{(aq)} \qquad E^θ = +0.40 V \)
Overall:
\(H_{2 (g)} + \frac{1}{2}O_{2 (g)} ⟶ H_2O_{(l)} \qquad E^θ_{cell} = +1.23 V \)
Non-hydrogen fuels
Methanol and other hydrogen-rich fuels can be used in fuel cells.
3