14.4 Combining half-cells
It is possible to combine any two half-cells together - it doesn't have to be the hydrogen electrode.
Combining zinc with copper half-cells
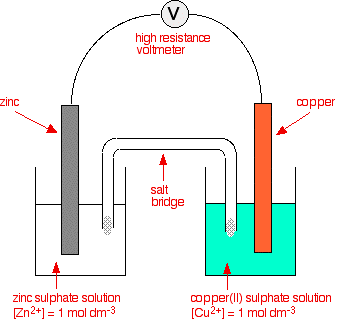
In the above example, two equilibria are set up:
\(Zn^{2+}_{(aq)} + 2e^- ⇌ Zn_{(s)} \qquad E^θ = -0.76 V \)
\(Cu^{2+}_{(aq)} + 2e^- ⇌ Cu_{(s)} \qquad E^θ = +0.34 V \)
The Eθ for zinc is more negative than that for copper, therefore zinc more readily gives up electrons to form ions than copper does.
Electron flow
Since there are more electrons on the zinc than on the copper, electrons will flow from the zinc to the copper.
As electrons are being lost from the zinc electrode (oxidation), zinc will give up more electrons to replace them in an attempt to restore the equilibrium (Le Chatelier's Principle).
As electrons are being gained by the copper electrode (reduction), copper ions will pick up the electrons to form copper atoms in an attempt to restore the equilibrium (Le Chatelier's Principle).
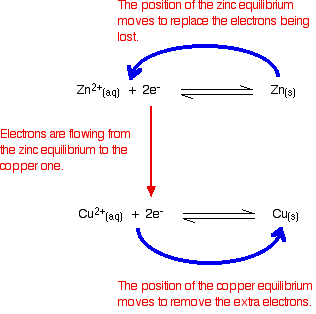
This essentially becomes two reactions as follows:
\(Zn_{(s)} ⟶ Zn^{2+}_{(aq)} + 2e^- \)
\(Cu^{2+}_{(aq)} + 2e^- ⟶ Cu_{(s)} \)
These two half-equations can be combined as follows:
\(Zn_{(s)} + Cu^{2+}_{(aq)} ⟶ Zn^{2+}_{(aq)} + Cu_{(s)} \)
Calculating standard cell potentials
The standard cell potential, Eθcell, can be calculated by finding the difference between the two standard electrode potentials as follows:
\(E^θ_{cell} = E^θ_{reduction} - E^θ_{oxidation} \)
In the above example, the copper electrode is being reduced, and the zinc electrode is being oxidised.
\(E^θ_{cell} = +0.34 - (- 0.76) = +1.1 V \)
Disproportionation reactions
Consider the two standard electrode potentials:
\(Cu^{2+}_{(aq)} + e^- ⇌ Cu^{+}_{(aq)} \qquad E^θ = +0.16 V \)
\(Cu^{+}_{(aq)} + e^- ⇌ Cu_{(s)} \qquad E^θ = +0.52 V \)
Combining the two half equestions:
\(2Cu^{+}_{(aq)} ⇌ Cu^{2+}_{(aq)} + Cu_{(s)} \)
In a disproportionation reaction, the same species is both oxidised and reduced. This is possible from combining half-cells (e.g. to copper).
Thermodynamic feasibility
A cell reaction is thermodynamically feasible (spontaneous) if Eθcell > 0.
Eθcell is directly proportional to the total entropy change and to ln K for a reaction. A positive Eθcell will lead to a positive total entropy change.
Limitations of predictions
Just because Eθcell indicates that a reaction is feasible (possible), it doesn't mean that it would necessarily happen.
This could be because the reaction has very high activation energy (kinetically slow/inhibition). Eθ values give no information about reaction rates.
The reaction might not be occurring in standard conditions. Eθ values apply to standard conditions only.
Combining zinc with copper half-cells

In the above example, two equilibria are set up:
\(Zn^{2+}_{(aq)} + 2e^- ⇌ Zn_{(s)} \qquad E^θ = -0.76 V \)
\(Cu^{2+}_{(aq)} + 2e^- ⇌ Cu_{(s)} \qquad E^θ = +0.34 V \)
The Eθ for zinc is more negative than that for copper, therefore zinc more readily gives up electrons to form ions than copper does.
Electron flow
Since there are more electrons on the zinc than on the copper, electrons will flow from the zinc to the copper.
As electrons are being lost from the zinc electrode (oxidation), zinc will give up more electrons to replace them in an attempt to restore the equilibrium (Le Chatelier's Principle).
As electrons are being gained by the copper electrode (reduction), copper ions will pick up the electrons to form copper atoms in an attempt to restore the equilibrium (Le Chatelier's Principle).

This essentially becomes two reactions as follows:
\(Zn_{(s)} ⟶ Zn^{2+}_{(aq)} + 2e^- \)
\(Cu^{2+}_{(aq)} + 2e^- ⟶ Cu_{(s)} \)
These two half-equations can be combined as follows:
\(Zn_{(s)} + Cu^{2+}_{(aq)} ⟶ Zn^{2+}_{(aq)} + Cu_{(s)} \)
Calculating standard cell potentials
The standard cell potential, Eθcell, can be calculated by finding the difference between the two standard electrode potentials as follows:
\(E^θ_{cell} = E^θ_{reduction} - E^θ_{oxidation} \)
In the above example, the copper electrode is being reduced, and the zinc electrode is being oxidised.
\(E^θ_{cell} = +0.34 - (- 0.76) = +1.1 V \)
Disproportionation reactions
Consider the two standard electrode potentials:
\(Cu^{2+}_{(aq)} + e^- ⇌ Cu^{+}_{(aq)} \qquad E^θ = +0.16 V \)
\(Cu^{+}_{(aq)} + e^- ⇌ Cu_{(s)} \qquad E^θ = +0.52 V \)
Combining the two half equestions:
\(2Cu^{+}_{(aq)} ⇌ Cu^{2+}_{(aq)} + Cu_{(s)} \)
In a disproportionation reaction, the same species is both oxidised and reduced. This is possible from combining half-cells (e.g. to copper).
Thermodynamic feasibility
A cell reaction is thermodynamically feasible (spontaneous) if Eθcell > 0.
Eθcell is directly proportional to the total entropy change and to ln K for a reaction. A positive Eθcell will lead to a positive total entropy change.
Limitations of predictions
Just because Eθcell indicates that a reaction is feasible (possible), it doesn't mean that it would necessarily happen.
This could be because the reaction has very high activation energy (kinetically slow/inhibition). Eθ values give no information about reaction rates.
The reaction might not be occurring in standard conditions. Eθ values apply to standard conditions only.
3