14.3 Standard electrode potentials
Measuring standard electrode potentials
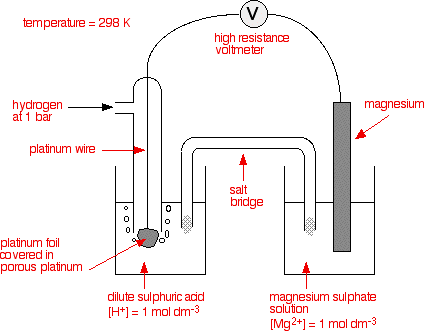
The standard hydrogen electrode is attached to the electrode system to be measured - for example, magnesium in a solution containing magnesium ions.
The connected system is known as a cell, and each electrode system is known as a half-cell. The cell generates a potential difference.
The salt bridge is included to complete the electrical circuit without adding further metal into the system. It is a glass tube filled with an electrolyte like potassium nitrate. The ends are plugged with cotton wool to stop too much mixing of the electrolyte with the solutions in the two beakers. A suitable electrolyte is chosen which does not react with the contents of either beaker.
The standard electrode potential, Eθ, refers to the potential difference between a standard hydrogen electrode and a half-cell, measured at the conditions of:
i) 298K temperature
ii) 100 kPa pressure of gases
iii) 1.00 mol dm-3 concentration of ions.
These conditions are important, because they affect the position of equilibrium. By standardising them, fair comparisons can be made between different systems.
In the above example, two equilibria are set up:
\(Mg^{2+}_{(aq)} + 2e^- ⇌ Mg_{(s)} \)
\(2H^+_{(aq)} + 2e^- ⇌ H_{2 (g)} \)
Magnesium has a greater tendency to form ions than hydrogen. The position of the equilibrium for magnesium will be further to the left of the equilibrium for hydrogen.
This means that there are more electrons on the magnesium than on the platinum, i.e. the magnesium electrode is more negative than the hydrogen electrode. The voltage measured would be -2.37 volts (the Eθ value).
The more negative the Eθ value, the further the equilibrium lies to the left - the more readily the element loses electrons and forms ions.
Conventional representation of cells and half-cells
The above example is represented as:
\(Pt | H_{2(g)} | 2H^+_{(aq)} || Mg^{2+}_{(aq)} | Mg_{(s)} \)
Non-metals and their ions
For non-metals, e.g. chlorine, the half-cell is built like a hydrogen electrode. Chlorine gas is bubbled over a platinum electrode, which is immersed in a solution containing chloride ions with a concentration of 1 mol dm-3.
Ions of the same element with different oxidation numbers
For ions of the same element with different oxidation numbers, e.g. Fe2+ and Fe3+, the half-cell has a platinum electrode inserted into a beaker containing a solution of both Fe2+ and Fe3+ ions (1 mol dm-3 for both).
The electrochemical series
The electrochemical series is a list of equilibria in the order of their standard electrode potentials, start with the most negative Eθ value at the top. It will either be provided in the formula book, or in the question, so there is no need to memorise any numbers.

The standard hydrogen electrode is attached to the electrode system to be measured - for example, magnesium in a solution containing magnesium ions.
The connected system is known as a cell, and each electrode system is known as a half-cell. The cell generates a potential difference.
The salt bridge is included to complete the electrical circuit without adding further metal into the system. It is a glass tube filled with an electrolyte like potassium nitrate. The ends are plugged with cotton wool to stop too much mixing of the electrolyte with the solutions in the two beakers. A suitable electrolyte is chosen which does not react with the contents of either beaker.
The standard electrode potential, Eθ, refers to the potential difference between a standard hydrogen electrode and a half-cell, measured at the conditions of:
i) 298K temperature
ii) 100 kPa pressure of gases
iii) 1.00 mol dm-3 concentration of ions.
These conditions are important, because they affect the position of equilibrium. By standardising them, fair comparisons can be made between different systems.
In the above example, two equilibria are set up:
\(Mg^{2+}_{(aq)} + 2e^- ⇌ Mg_{(s)} \)
\(2H^+_{(aq)} + 2e^- ⇌ H_{2 (g)} \)
Magnesium has a greater tendency to form ions than hydrogen. The position of the equilibrium for magnesium will be further to the left of the equilibrium for hydrogen.
This means that there are more electrons on the magnesium than on the platinum, i.e. the magnesium electrode is more negative than the hydrogen electrode. The voltage measured would be -2.37 volts (the Eθ value).
The more negative the Eθ value, the further the equilibrium lies to the left - the more readily the element loses electrons and forms ions.
Conventional representation of cells and half-cells
The above example is represented as:
\(Pt | H_{2(g)} | 2H^+_{(aq)} || Mg^{2+}_{(aq)} | Mg_{(s)} \)
Non-metals and their ions
For non-metals, e.g. chlorine, the half-cell is built like a hydrogen electrode. Chlorine gas is bubbled over a platinum electrode, which is immersed in a solution containing chloride ions with a concentration of 1 mol dm-3.
Ions of the same element with different oxidation numbers
For ions of the same element with different oxidation numbers, e.g. Fe2+ and Fe3+, the half-cell has a platinum electrode inserted into a beaker containing a solution of both Fe2+ and Fe3+ ions (1 mol dm-3 for both).
The electrochemical series
The electrochemical series is a list of equilibria in the order of their standard electrode potentials, start with the most negative Eθ value at the top. It will either be provided in the formula book, or in the question, so there is no need to memorise any numbers.
3