14.2 Standard hydrogen electrode
The standard hydrogen electrode is shown below.
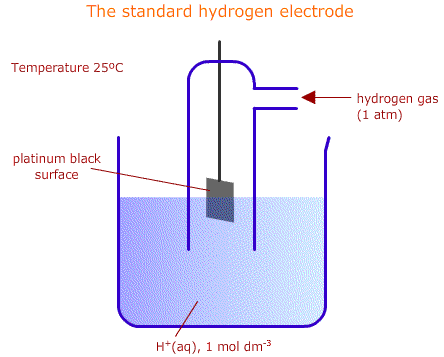
As the hydrogen gas flows over the platinum catalyst, an equilibrium is set up between hydrogen molecules and hydrogen ions in solution.
\(2H^+_{(aq)} + 2e^- ⇌ H_{2 (g)} \)
This is the equilibrium that all other systems are compared with.
Standard conditions
Because the position of equlibrium is affected by certain conditions, these must also be standardised so that a fair comparison can be made.
The standard conditions are a temperature of 298K (25°C), a gas pressure of 100 kPa (1 bar or 1 atmosphere) and ionic concentration in solution of 1 mol dm-3.

As the hydrogen gas flows over the platinum catalyst, an equilibrium is set up between hydrogen molecules and hydrogen ions in solution.
\(2H^+_{(aq)} + 2e^- ⇌ H_{2 (g)} \)
This is the equilibrium that all other systems are compared with.
Standard conditions
Because the position of equlibrium is affected by certain conditions, these must also be standardised so that a fair comparison can be made.
The standard conditions are a temperature of 298K (25°C), a gas pressure of 100 kPa (1 bar or 1 atmosphere) and ionic concentration in solution of 1 mol dm-3.
3