14.1 Introduction to redox equilibria
As a reminder, redox occurs when reduction and oxidation occurs at the same time.
Oxidation refers to the loss of electrons. This leads to an increase in the oxidation number.
Reduction refers to the gain of electrons. This leads to a decrease in the oxidation number.
Remember: OILRIG - Oxidation Is Loss, Reduction Is Gain (of electrons)
Reactivities of metals and equilibria
Consider placing magnesium in water - Magnesium will form hydrated ions in solution:
\(Mg_{(s)} ⟶ Mg^{2+}_{(aq)} + 2e^- \)
The electrons which are released will build up on the magnesium, and the positive ions which form will stay close to the surface of the magesium due to attaction to the negative charge on the metal. Some of the magnesium ions will be attracted enough that they pick up their electrons again, and reform magnesium atoms.
Thus, a dynamic equilibrium is set up:
\(Mg^{2+}_{(aq)} + 2e^- ⇌ Mg_{(s)} \)
Now consider if you had used copper instead. Copper is less reactive than magnesium, therefore less likely to form ions. Any ions that form are more likely to pick up their electrons again to reform copper atoms. Thus, there will be fewer electrons on the metal, and fewer ions in solution.
\(Cu^{2+}_{(aq)} + 2e^- ⇌ Cu_{(s)} \)
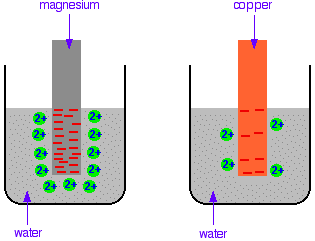
The position of equilibrium for magnesium lies further to the left than for copper.
Electrode potentials
Electrode potentials are a way of attempting to quantify the difference in the position of equilibrium.
Why not just measure the potential difference between the metal and the solution? In practice, this is impossible to do, because if you insert a probe into the solution, a similar equilibrium would also form around the probe, so you are not actually measuring the potential difference between the metal and solution. You would be measuring an interaction between the metal and the probe.
The potential difference between the two systems (magnesium and copper) quantifies the difference in the position of equilibrium. There is no need to measure the absolute potential difference between the metal and solution. It is enough to be able to compare one system with another.
The standardised system used for comparison is known as a reference electrode - the standard hydrogen electrode.
Oxidation refers to the loss of electrons. This leads to an increase in the oxidation number.
Reduction refers to the gain of electrons. This leads to a decrease in the oxidation number.
Remember: OILRIG - Oxidation Is Loss, Reduction Is Gain (of electrons)
Reactivities of metals and equilibria
Consider placing magnesium in water - Magnesium will form hydrated ions in solution:
\(Mg_{(s)} ⟶ Mg^{2+}_{(aq)} + 2e^- \)
The electrons which are released will build up on the magnesium, and the positive ions which form will stay close to the surface of the magesium due to attaction to the negative charge on the metal. Some of the magnesium ions will be attracted enough that they pick up their electrons again, and reform magnesium atoms.
Thus, a dynamic equilibrium is set up:
\(Mg^{2+}_{(aq)} + 2e^- ⇌ Mg_{(s)} \)
Now consider if you had used copper instead. Copper is less reactive than magnesium, therefore less likely to form ions. Any ions that form are more likely to pick up their electrons again to reform copper atoms. Thus, there will be fewer electrons on the metal, and fewer ions in solution.
\(Cu^{2+}_{(aq)} + 2e^- ⇌ Cu_{(s)} \)

The position of equilibrium for magnesium lies further to the left than for copper.
Electrode potentials
Electrode potentials are a way of attempting to quantify the difference in the position of equilibrium.
Why not just measure the potential difference between the metal and the solution? In practice, this is impossible to do, because if you insert a probe into the solution, a similar equilibrium would also form around the probe, so you are not actually measuring the potential difference between the metal and solution. You would be measuring an interaction between the metal and the probe.
The potential difference between the two systems (magnesium and copper) quantifies the difference in the position of equilibrium. There is no need to measure the absolute potential difference between the metal and solution. It is enough to be able to compare one system with another.
The standardised system used for comparison is known as a reference electrode - the standard hydrogen electrode.
3