#7.2.1
Structure and formulae of alkenes
Alkenes are hydrocarbons with a double carbon-carbon bond. The general formula for the homologous series of alkenes is CnH2n.
Alkene molecules are unsaturated because they contain two fewer hydrogen atoms than the alkane with the same number of carbon atoms.
The first four members of the homologous series of alkenes are ethene, propene, butene and pentene.
Alkene molecules can be represented in the following forms:
C3H6 or
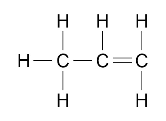
Students do not need to know the names of individual alkenes other than ethene, propene, butene and pentene.
#7.2.2
Reactions of alkenes
Alkenes are hydrocarbons with the functional group C=C.
It is the generality of reactions of functional groups that determine the reactions of organic compounds.
Alkenes react with oxygen in combustion reactions in the same way as other hydrocarbons, but they tend to burn in air with smoky flames because of incomplete combustion.
Alkenes react with hydrogen, water and the halogens, by the addition of atoms across the carbon-carbon double bond so that the double bond becomes a single carbon-carbon bond.
Students should be able to:
- describe the reactions and conditions for the addition of hydrogen, water and halogens to alkenes
- draw fully displayed structural formulae of the first four members of the alkenes and the products of their addition reactions with hydrogen, water, chlorine, bromine and iodine.
#7.2.3
Alcohols
Alcohols contain the functional group –OH.
Methanol, ethanol, propanol and butanol are the first four members of a homologous series of alcohols.
Alcohols can be represented in the following forms:
CH3CH2OH or
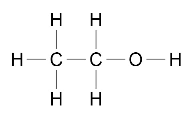
Students should be able to:
- describe what happens when any of the first four alcohols react with sodium, burn in air, are added to water, react with an oxidising agent
- recall the main uses of these alcohols.
Aqueous solutions of ethanol are produced when sugar solutions are fermented using yeast.
Students should know the conditions used for fermentation of sugar using yeast.
Students should be able to recognise alcohols from their names or from given formulae.
Students do not need to know the names of individual alcohols other than methanol, ethanol, propanol and butanol.
Students are not expected to write balanced chemical equations for the reactions of alcohols other than for combustion reactions.
#7.2.4
Carboxylic acids
Carboxylic acids have the functional group –COOH.
The first four members of a homologous series of carboxylic acids are methanoic acid, ethanoic acid, propanoic acid and butanoicacid.
The structures of carboxylic acids can be represented in the following forms:
CH3COOH or
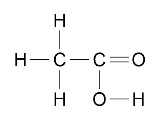
Students should be able to:
- describe what happens when any of the first four carboxylic acids react with carbonates, dissolve in water, react with alcohols
- explain why carboxylic acids are weak acids in terms of ionisation and pH (see Strong and weak acids).
Students should be able to recognise carboxylic acids from their names or from given formulae.
Students do not need to know the names of individual carboxylic acids other than methanoic acid, ethanoic acid, propanoic acid and butanoic acid.
Students are not expected to write balanced chemical equations for the reactions of carboxylic acids.
Students do not need to know the names of esters other than ethyl ethanoate.