#7.1.1
Crude oil, hydrocarbons and alkanes
Crude oil is a finite resource found in rocks. Crude oil is the remains of an ancient biomass consisting mainly of plankton that was buried in mud.
Crude oil is a mixture of a very large number of compounds. Most of the compounds in crude oil are hydrocarbons, which are molecules made up of hydrogen and carbon atoms only.
Most of the hydrocarbons in crude oil are hydrocarbons called alkanes. The general formula for the homologous series of alkanes is CnH2n+2.
The first four members of the alkanes are methane, ethane, propane and butane.
Alkane molecules can be represented in the following forms:
C2H6 or
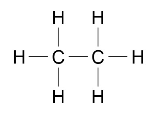
Students should be able to recognise substances as alkanes given their formulae in these forms.
Students do not need to know the names of specific alkanes other than methane, ethane, propane and butane.
#7.1.2
Fractional distillation and petrochemicals
The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by fractional distillation.
The fractions can be processed to produce fuels and feedstock for the petrochemical industry.
Many of the fuels on which we depend for our modern lifestyle, such as petrol, diesel oil, kerosene, heavy fuel oil and liquefied petroleum gases, are produced from crude oil.
Many useful materials on which modern life depends are produced by the petrochemical industry, such as solvents, lubricants, polymers, detergents.
The vast array of natural and synthetic carbon compounds occur due to the ability of carbon atoms to form families of similar compounds.
Students should be able to explain how fractional distillation works in terms of evaporation and condensation.
Knowledge of the names of other specific fractions or fuels is not required.
#7.1.3
Properties of hydrocarbons
Some properties of hydrocarbons depend on the size of their molecules, including boiling point, viscosity and flammability. These properties influence how hydrocarbons are used as fuels.
Students should be able to recall how boiling point, viscosity and flammability change with increasing molecular size.
The combustion of hydrocarbon fuels releases energy. During combustion, the carbon and hydrogen in the fuels are oxidised. The complete combustion of a hydrocarbon produces carbon dioxide and water.
Students should be able to write balanced equations for the complete combustion of hydrocarbons with a given formula.
Knowledge of trends in properties of hydrocarbons is limited to:
- boiling points
- viscosity
- flammability.
#7.1.4
Cracking and alkenes
Hydrocarbons can be broken down (cracked) to produce smaller, more useful molecules.
Cracking can be done by various methods including catalytic cracking and steam cracking.
Students should be able to describe in general terms the conditions used for catalytic cracking and steam cracking.
The products of cracking include alkanes and another type of hydrocarbon called alkenes.
Alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes.
Students should be able to recall the colour change when bromine water reacts with an alkene.
There is a high demand for fuels with small molecules and so some of the products of cracking are useful as fuels.
Alkenes are used to produce polymers and as starting materials for the production of many other chemicals.
Students should be able to balance chemical equations as examples of cracking given the formulae of the reactants and products.
Students should be able to give examples to illustrate the usefulness of cracking. They should also be able to explain how modern life depends on the uses of hydrocarbons.