#7.3.1
Addition polymerisation
Alkenes can be used to make polymers such as poly(ethene) and poly(propene) by addition polymerisation.
In addition polymerisation reactions, many small molecules (monomers) join together to form very large molecules (polymers).
For example:
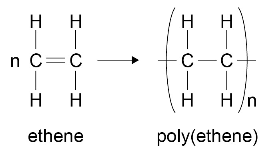
In addition polymers the repeating unit has the same atoms as the monomer because no other molecule is formed in the reaction.
Students should be able to:
- recognise addition polymers and monomers from diagrams in the forms shown and from the presence of the functional group C=C in the monomers
- draw diagrams to represent the formation of a polymer from a given alkene monomer
- relate the repeating unit to the monomer.
#7.3.2
Condensation polymerisation
Condensation polymerisation involves monomers with two functional groups. When these types of monomers react they join together, usually losing small molecules such as water, and so the reactions are called condensation reactions.
The simplest polymers are produced from two different monomers with two of the same functional groups on each monomer.
For example:
ethanediol
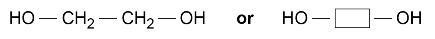
and hexanedioic acid
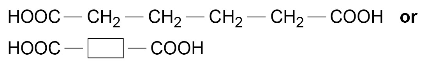
polymerise to produce a polyester:
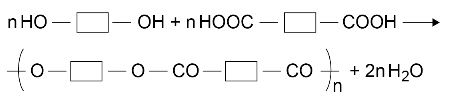
Students should be able to explain the basic principles of condensation polymerisation by reference to the functional groups in the monomers and the repeating units in the polymers.
#7.3.3
Amino acids
Amino acids have two different functional groups in a molecule. Amino acids react by condensation polymerisation to produce polypeptides.
For example: glycine is H2NCH2COOH and polymerises to produce the polypeptide
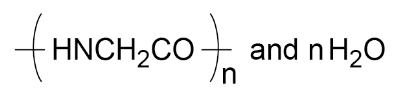
Different amino acids can be combined in the same chain to produce proteins.
#7.3.4
DNA (deoxyribonucleic acid) and other naturally occurring polymers
DNA (deoxyribonucleic acid) is a large molecule essential for life. DNA encodes genetic instructions for the development and functioning of living organisms and viruses.
Most DNA molecules are two polymer chains, made from four different monomers called nucleotides, in the form of a double helix. Other naturally occurring polymers important for life include proteins, starch and cellulose.
Students should be able to name the types of monomers from which these naturally occurring polymers are made.