12.8 Acid-base indicators
Indicators are used to identify the end point of a titration, and change colour over a narrow range of pH.
Methyl orange changes from red (low pH) to yellow (high pH) at around pH 3.1-4.4.
Phenolphthalein changes from colourless (low pH) to pink (high pH) at around pH 8.3-10.
You should choose an indicator which changes colour at or near the equivalence point.
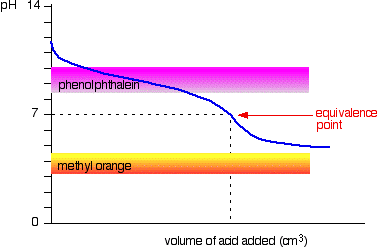
Adding strong acid into strong base
For example: adding hydrochloric acid to sodium hydroxide:

Neither indicator changes colour at the equivalence point here, but because the graph is so steep, either could be used to detect the end point of the titration.
Adding strong acid into weak base
For example: adding hydrochloric acid to ammonia solution:

Methyl orange changes colour close to the equivalence point here, so is the appropriate indicator to use.
Adding weak acid into strong base
For example: adding ethanoic acid to sodium hydroxide:

Phenolphthalein changes colour at the equivalence point here, so is the appropriate indicator to use.
Adding weak acid into weak base
For example: adding ethanoic acid and ammonia solution:
Neither indicator would be appropriate here. Note that there is no steep section on this graph, so it is difficult to do a titration of a weak acid against a weak base.
Explaining the colour change
Indicators can be thought of as weak acids, HA. The following equilibrium is observed:
\(HA_{(aq)} ⇌ H^+_{(aq)} + A^-_{(aq)} \)
The HA and A- forms of the indicator have different colours.
For methyl orange, the HA form is red, and the A- form is yellow.
For phenolphthalein, the HA form is colourless, and the A- form is pink.
In acidic conditions, where there is an excess of H+ ions, the equilibrium shifts to the left, so that there is an excess of the [HA] form of the indicator.
In alkaline conditions, where there could be an excess of OH- ions, H+ ions will react with OH- ions and be removed. Hence equilibrium shifts to the right, so that there is an excess of the A- form of the indicator.
Methyl orange changes from red (low pH) to yellow (high pH) at around pH 3.1-4.4.
Phenolphthalein changes from colourless (low pH) to pink (high pH) at around pH 8.3-10.
You should choose an indicator which changes colour at or near the equivalence point.
Adding strong acid into strong base
For example: adding hydrochloric acid to sodium hydroxide:

Neither indicator changes colour at the equivalence point here, but because the graph is so steep, either could be used to detect the end point of the titration.
Adding strong acid into weak base
For example: adding hydrochloric acid to ammonia solution:

Methyl orange changes colour close to the equivalence point here, so is the appropriate indicator to use.
Adding weak acid into strong base
For example: adding ethanoic acid to sodium hydroxide:

Phenolphthalein changes colour at the equivalence point here, so is the appropriate indicator to use.
Adding weak acid into weak base
For example: adding ethanoic acid and ammonia solution:

Neither indicator would be appropriate here. Note that there is no steep section on this graph, so it is difficult to do a titration of a weak acid against a weak base.
Explaining the colour change
Indicators can be thought of as weak acids, HA. The following equilibrium is observed:
\(HA_{(aq)} ⇌ H^+_{(aq)} + A^-_{(aq)} \)
The HA and A- forms of the indicator have different colours.
For methyl orange, the HA form is red, and the A- form is yellow.
For phenolphthalein, the HA form is colourless, and the A- form is pink.
In acidic conditions, where there is an excess of H+ ions, the equilibrium shifts to the left, so that there is an excess of the [HA] form of the indicator.
In alkaline conditions, where there could be an excess of OH- ions, H+ ions will react with OH- ions and be removed. Hence equilibrium shifts to the right, so that there is an excess of the A- form of the indicator.
3