12.7 pH titration curves
The equivalence point of a titration is when the acid and base have been mixed in exactly the correct proportions according to the equation.
For hydrochloric acid and sodium hydroxide:
\(HCl_{(aq)} + NaOH_{(aq)} → NaCl_{(aq)} + H_2O_{(l)} \)
Because the ratio is 1:1, the equivalence point will have been reached when 25 cm3 of 1 mol dm-3 HCl is added to 25 cm3 of 1 mol dm-3 NaOH.
Adding strong acid into strong base
For example: adding hydrochloric acid to sodium hydroxide:
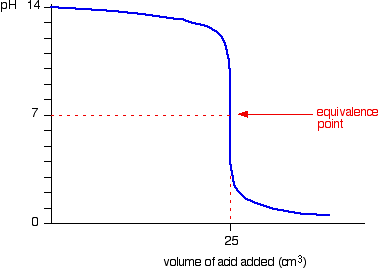
The pH decreases very slightly until very near the equivalence point, when it decreases sharply. The equivalence point is at 7 because sodium chloride and water are neutral.
Adding strong acid into weak base
For example: adding hydrochloric acid to ammonia solution:
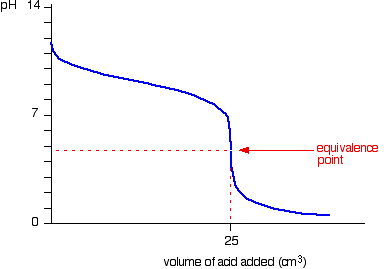
Initially, the pH falls rapidly as acid is being added. Soon, the pH decreases slowly as more acid is added, because a buffer solution has been set up, since there is a mixture of ammonia and ammonium chloride. The equivalence point is around 5 because ammonium chloride is slightly acidic. Past the equivalence point, there is an excess of strong acid, so the graph behaves like adding a strong acid to a strong base.
Adding weak acid into strong base
For example: adding ethanoic acid to sodium hydroxide:
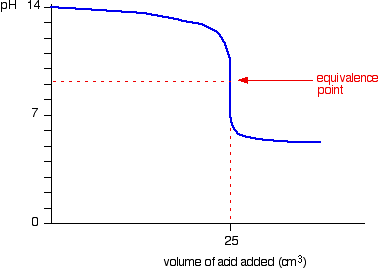
Initially, there is an excess of strong base, so the graph behaves like adding a strong acid to a strong base. The equivalence point is around 9 because sodium ethanoate is slightly alkaline. Past the equivalence point, the pH decreases slowly as more acid is added, because a buffer solution has been set up, since there is a mixture of ethanoic acid and sodium ethanoate. Since ethanoic acid is a weak acid, the pH won't drop significantly.
Adding weak acid into weak base
For example: adding ethanoic acid and ammonia solution:
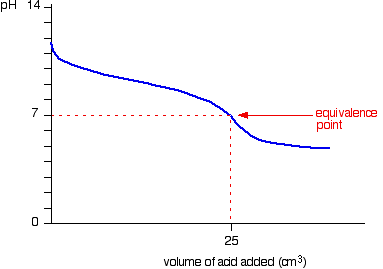
This is a combination of the graph of adding strong acid into weak base (before the equivalence point) and the graph of adding weak acid into strong base (past the equivalence point). Note that there is no rapid drop in pH here (no steep section).
Credit to Chemguide for the above graphs
Calculating Ka from the pH at the half equivalence point
The half equivalence point is the point at which half the weak acid is neutralised. The Ka of a weak acid can be calculated by finding the pH at the half equivalence point.
\(K_a = \dfrac{[H^+][A^-]}{[HA]} \)
At the half equivalence point, [HA] = [A-], therefore:
\(K_a = \dfrac{[H^+]\cancel{[A^-]}}{\cancel{[HA]}} \)
\(K_a = [H^+] \)
For hydrochloric acid and sodium hydroxide:
\(HCl_{(aq)} + NaOH_{(aq)} → NaCl_{(aq)} + H_2O_{(l)} \)
Because the ratio is 1:1, the equivalence point will have been reached when 25 cm3 of 1 mol dm-3 HCl is added to 25 cm3 of 1 mol dm-3 NaOH.
Adding strong acid into strong base
For example: adding hydrochloric acid to sodium hydroxide:

The pH decreases very slightly until very near the equivalence point, when it decreases sharply. The equivalence point is at 7 because sodium chloride and water are neutral.
Adding strong acid into weak base
For example: adding hydrochloric acid to ammonia solution:

Initially, the pH falls rapidly as acid is being added. Soon, the pH decreases slowly as more acid is added, because a buffer solution has been set up, since there is a mixture of ammonia and ammonium chloride. The equivalence point is around 5 because ammonium chloride is slightly acidic. Past the equivalence point, there is an excess of strong acid, so the graph behaves like adding a strong acid to a strong base.
Adding weak acid into strong base
For example: adding ethanoic acid to sodium hydroxide:

Initially, there is an excess of strong base, so the graph behaves like adding a strong acid to a strong base. The equivalence point is around 9 because sodium ethanoate is slightly alkaline. Past the equivalence point, the pH decreases slowly as more acid is added, because a buffer solution has been set up, since there is a mixture of ethanoic acid and sodium ethanoate. Since ethanoic acid is a weak acid, the pH won't drop significantly.
Adding weak acid into weak base
For example: adding ethanoic acid and ammonia solution:

This is a combination of the graph of adding strong acid into weak base (before the equivalence point) and the graph of adding weak acid into strong base (past the equivalence point). Note that there is no rapid drop in pH here (no steep section).
Credit to Chemguide for the above graphs
Calculating Ka from the pH at the half equivalence point
The half equivalence point is the point at which half the weak acid is neutralised. The Ka of a weak acid can be calculated by finding the pH at the half equivalence point.
\(K_a = \dfrac{[H^+][A^-]}{[HA]} \)
At the half equivalence point, [HA] = [A-], therefore:
\(K_a = \dfrac{[H^+]\cancel{[A^-]}}{\cancel{[HA]}} \)
\(K_a = [H^+] \)
3