#2.2.1
The three states of matter
The three states of matter are solid, liquid and gas. Melting and freezing take place at the melting point, boiling and condensing take place at the boiling point.
The three states of matter can be represented by a simple model. In this model, particles are represented by small solid spheres. Particle theory can help to explain melting, boiling, freezing and condensing.
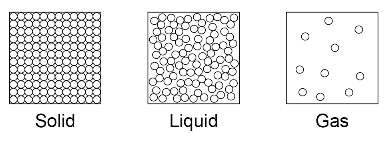
The amount of energy needed to change state from solid to liquid and from liquid to gas depends on the strength of the forces between the particles of the substance. The nature of the particles involved depends on the type of bonding and the structure of the substance. The stronger the forces between the particles the higher the melting point and boiling point of the substance.
Limitations of the simple model above include that in the model there are no forces, that all particles are represented as spheres and that the spheres are solid.
Students should be able to:
- predict the states of substances at different temperatures given appropriate data
- explain the different temperatures at which changes of state occur in terms of energy transfers and types of bonding
- recognise that atoms themselves do not have the bulk properties of materials
- explain the limitations of the particle theory in relation to changes of state when particles are represented by solid inelastic spheres which have no forces between them.
#2.2.2
State symbols
In chemical equations, the three states of matter are shown as (s), (l) and (g), with (aq) for aqueous solutions.
Students should be able to include appropriate state symbols in chemical equations for the reactions in this specification.
#2.2.3
Properties of ionic compounds
Ionic compounds have regular structures (giant ionic lattices) in which there are strong electrostatic forces of attraction in all directions between oppositely charged ions.
These compounds have high melting points and high boiling points because of the large amounts of energy needed to break the many strong bonds.
When melted or dissolved in water, ionic compounds conduct electricity because the ions are free to move and so charge can flow.
Knowledge of the structures of specific ionic compounds other than sodium chloride is not required.
#2.2.4
Properties of small molecules
Substances that consist of small molecules are usually gases or liquids that have relatively low melting points and boiling points.
These substances have only weak forces between the molecules (intermolecular forces). It is these intermolecular forces that are overcome, not the covalent bonds, when the substance melts or boils.
The intermolecular forces increase with the size of the molecules, so larger molecules have higher melting and boiling points.
These substances do not conduct electricity because the molecules do not have an overall electric charge.
Students should be able to use the idea that intermolecular forces are weak compared with covalent bonds to explain the bulk properties of molecular substances.
#2.2.5
Polymers
Polymers have very large molecules. The atoms in the polymer molecules are linked to other atoms by strong covalent bonds. The intermolecular forces between polymer molecules are relatively strong and so these substances are solids at room temperature.
Students should be able to recognise polymers from diagrams showing their bonding and structure.
#2.2.6
Giant covalent structures
Substances that consist of giant covalent structures are solids with very high melting points. All of the atoms in these structures are linked to other atoms by strong covalent bonds. These bonds must be overcome to melt or boil these substances. Diamond and graphite (forms of carbon) and silicon dioxide (silica) are examples of giant covalent structures.
Students should be able to recognise giant covalent structures from diagrams showing their bonding and structure.
#2.2.7
Properties of metals and alloys
Metals have giant structures of atoms with strong metallic bonding. This means that most metals have high melting and boiling points.
In pure metals, atoms are arranged in layers, which allows metals to be bent and shaped. Pure metals are too soft for many uses and so are mixed with other metals to make alloys which are harder.
Students should be able to explain why alloys are harder than pure metals in terms of distortion of the layers of atoms in the structure of a pure metal.
#2.2.8
Metals as conductors
Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. Metals are good conductors of thermal energy because energy is transferred by the delocalised electrons.