#2.1.1
Chemical bonds
There are three types of strong chemical bonds: ionic, covalent and metallic. For ionic bonding the particles are oppositely charged ions. For covalent bonding the particles are atoms which share pairs of electrons. For metallic bonding the particles are atoms which share delocalised electrons.
Ionic bonding occurs in compounds formed from metals combined with non-metals.
Covalent bonding occurs in most non-metallic elements and in compounds of non-metals.
Metallic bonding occurs in metallic elements and alloys.
Students should be able to explain chemical bonding in terms of electrostatic forces and the transfer or sharing of electrons.
#2.1.2
Ionic bonding
When a metal atom reacts with a non-metal atom electrons in the outer shell of the metal atom are transferred. Metal atoms lose electrons to become positively charged ions. Non-metal atoms gain electrons to become negatively charged ions. The ions produced by metals in Groups 1 and 2 and by non-metals in Groups 6 and 7 have the electronic structure of a noble gas (Group 0).
The electron transfer during the formation of an ionic compound can be represented by a dot and cross diagram, eg for sodium chloride
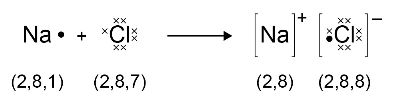
Students should be able to draw dot and cross diagrams for ionic compounds formed by metals in Groups 1 and 2 with non-metals in Groups 6 and 7.
The charge on the ions produced by metals in Groups 1 and 2 and by non-metals in Groups 6 and 7 relates to the group number of the element in the periodic table.
Students should be able to work out the charge on the ions of metals and non-metals from the group number of the element, limited to the metals in Groups 1 and 2, and non-metals in Groups 6 and 7.
#2.1.3
Ionic compounds
An ionic compound is a giant structure of ions. Ionic compounds are held together by strong electrostatic forces of attraction between oppositely charged ions. These forces act in all directions in the lattice and this is called ionic bonding.
The structure of sodium chloride can be represented in the following forms:
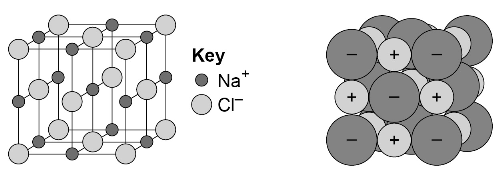
Students should be able to:
- deduce that a compound is ionic from a diagram of its structure in one of the specified forms
- describe the limitations of using dot and cross, ball and stick, two and three-dimensional diagrams to represent a giant ionic structure
- work out the empirical formula of an ionic compound from a given model or diagram that shows the ions in the structure.
Students should be familiar with the structure of sodium chloride but do not need to know the structures of other ionic compounds.
#2.1.4
Covalent bonding
When atoms share pairs of electrons, they form covalent bonds. These bonds between atoms are strong.
Covalently bonded substances may consist of small molecules.
Students should be able to recognise common substances that consist of small molecules from their chemical formula.
Some covalently bonded substances have very large molecules, such as polymers.
Some covalently bonded substances have giant covalent structures, such as diamond and silicon dioxide.
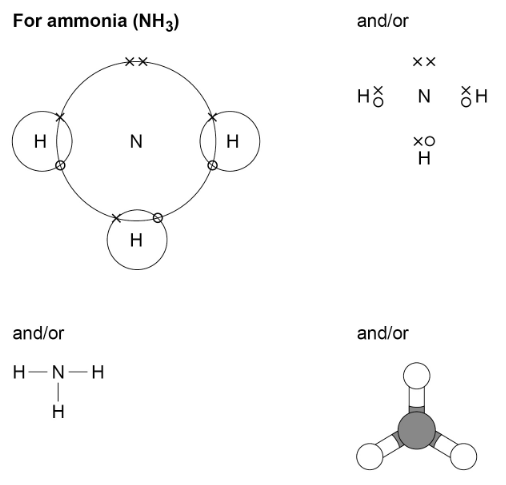
The covalent bonds in molecules and giant structures can be represented in the following forms:
Polymers can be represented in the form:
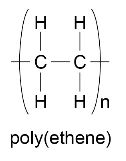
where n is a large number.
Students should be able to:
- draw dot and cross diagrams for the molecules of hydrogen, chlorine, oxygen, nitrogen, hydrogen chloride, water, ammonia and methane
- represent the covalent bonds in small molecules, in the repeating units of polymers and in part of giant covalent structures, using a line to represent a single bond
- describe the limitations of using dot and cross, ball and stick, two and three-dimensional diagrams to represent molecules or giant structures
- deduce the molecular formula of a substance from a given model or diagram in these forms showing the atoms and bonds in the molecule.
#2.1.5
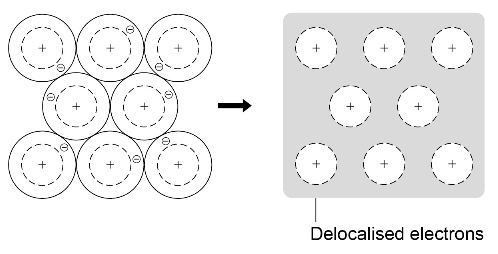
Metallic bonding
Metals consist of giant structures of atoms arranged in a regular pattern.
The electrons in the outer shell of metal atoms are delocalised and so are free to move through the whole structure. The sharing of delocalised electrons gives rise to strong metallic bonds. The bonding in metals may be represented in the following form: