#18.1
understand that the bonding in benzene has been represented using the Kekulé and the delocalised model, the latter in terms of overlap of p-orbitals to form π-bonds
#18.2
understand that evidence for the delocalised model of the bonding in benzene is provided by data from enthalpy changes of hydrogenation and carbon-carbon bond lengths
Students may represent the structure of benzene as:
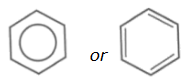
as appropriate in equations and mechanisms.
#18.3
understand why benzene is resistant to bromination, compared with alkenes, in terms of delocalisation of π-bonds in benzene and the localised electron density of the π-bond in alkenes
#18.4
understand the reactions of benzene with:
i) oxygen in air (combustion with a smoky flame)
ii) bromine, in the presence of a catalyst
iii) a mixture of concentrated nitric and sulfuric acids
iv) halogenoalkanes and acyl chlorides with aluminium chloride as catalyst (Friedel-Crafts reaction)
#18.5
understand the mechanism of the electrophilic substitution reactions of benzene (halogenation, nitration and Friedel-Crafts reactions), including the generation of the electrophile
#18.6
understand the reaction of phenol with bromine water
#18.7
understand reasons for the relative ease of bromination of phenol, compared to benzene
#18.8
be able to identify:
i) the amine and amide functional groups
ii) molecules that are amino acids
#18.9
understand the reactions of primary aliphatic amines, using butylamine as an example, with:
i) water to form an alkaline solution
ii) acids to form salts
iii) ethanoyl chloride
iv) halogenoalkanes
v) copper (II) ions to form complex ions
#18.10
understand reasons for the difference in basicity of ammonia, primary aliphatic and primary aromatic amines given suitable data
#18.11
understand, in terms of reagents and general reaction conditions, the preparation of primary aliphatic amines:
i) from halogenoalkanes
ii) by the reduction of nitriles
#18.12
know that aromatic nitro-compounds can be reduced, using tin and concentrated hydrochloric acid, to form amines
#18.13
understand that amides can be prepared from acyl chlorides
#18.14
know that the formation of a polyamide is a condensation polymerisation reaction
#18.15
be able to draw the structural formulae of the repeat units of condensation polymers formed by reactions between:
i) dicarboxylic acids and diols
ii) dicarboxylic acids and diamines
iii) amino acids
#18.16
understand the properties of 2-amino acids, including:
i) acidity and basicity in solution, as a result of the formation of zwitterions
ii) effect of aqueous solutions on plane-polarised monochromatic light
#18.17
understand that the peptide bond in proteins:
i) is formed when amino acids combine, by condensation polymerisation
ii) can be hydrolysed to form the constituent amino acids, which can be separated by chromatography
#18P15
CORE PRACTICAL 15: Analysis of some inorganic and organic unknowns
#18.18
be able to deduce the empirical formulae, molecular formulae and structural formulae of compounds from data obtained from combustion analysis, elemental percentage composition, characteristic reactions of functional groups, infrared spectra, mass spectra and nuclear magnetic resonance
#18.19
be able to plan reaction schemes, of up to four steps, to form both familiar and unfamiliar compounds
#18.20
understand methods of increasing the length of the carbon chain in a molecule by the use of magnesium to form Grignard reagents and the reactions of the latter with carbon dioxide and with carbonyl compounds in dry ether
#18.21
be able to select and justify suitable practical procedures for carrying out reactions involving compounds with functional groups included in the specification, including identifying appropriate control measures to reduce risk, based on data about hazards
#18.22
understand the following techniques used in the preparation and purification of organic compounds:
i) refluxing
ii) purification by washing
iii) solvent extraction
iv) recrystallisation
v) drying
vi) distillation, including steam distillation
vii) melting temperature determination
viii) boiling temperature determination
#18P16
CORE PRACTICAL 16: The preparation of aspirin