#4.1.1
The structure of an atom
Atoms are very small, having a radius of about 1 × 10-10 metres.
The basic structure of an atom is a positively charged nucleus composed of both protons and neutrons surrounded by negatively charged electrons.
The radius of a nucleus is less than 1/10 000 of the radius of an atom. Most of the mass of an atom is concentrated in the nucleus.
The electrons are arranged at different distances from the nucleus (different energy levels). The electron arrangements may change with the absorption of electromagnetic radiation (move further from the nucleus; a higher energy level) or by the emission of electromagnetic radiation (move closer to the nucleus; a lower energy level).
#4.1.2
Mass number, atomic number and isotopes
In an atom the number of electrons is equal to the number of protons in the nucleus. Atoms have no overall electrical charge.
All atoms of a particular element have the same number of protons. The number of protons in an atom of an element is called its atomic number.
The total number of protons and neutrons in an atom is called its mass number.
Atoms can be represented as shown in this example:
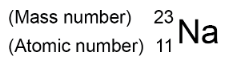
Atoms of the same element can have different numbers of neutrons; these atoms are called isotopes of that element.
Atoms turn into positive ions if they lose one or more outer electron(s).
Students should be able to relate differences between isotopes to differences in conventional representations of their identities, charges and masses.
#4.1.3
The development of the model of the atom (common with chemistry)
New experimental evidence may lead to a scientific model being changed or replaced.
Before the discovery of the electron, atoms were thought to be tiny spheres that could not be divided.
The discovery of the electron led to the plum pudding model of the atom. The plum pudding model suggested that the atom is a ball of positive charge with negative electrons embedded in it.
The results from the alpha particle scattering experiment led to the conclusion that the mass of an atom was concentrated at the centre (nucleus) and that the nucleus was charged. This nuclear model replaced the plum pudding model.
Niels Bohr adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observations.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
Students should be able to describe:
- why the new evidence from the scattering experiment led to a change in the atomic model
- the difference between the plum pudding model of the atom and the nuclear model of the atom.
Details of experimental work supporting the Bohr model are not required.
Details of Chadwick’s experimental work are not required.