#6.1.1
Calculating rates of reactions
The rate of a chemical reaction can be found by measuring the quantity of a reactant used or the quantity of product formed over time:
\(\text{mean rate of reaction} = \dfrac{\text{quantity of reactant used}}{\text{time taken}} \)
\(\text{mean rate of reaction} = \dfrac{\text{quantity of product formed}}{\text{time taken}} \)
The quantity of reactant or product can be measured by the mass in grams or by a volume in cm3.
The units of rate of reaction may be given as g/s or cm3/s.
For the Higher Tier, students are also required to use quantity of reactants in terms of moles and units for rate of reaction in mol/s.
Students should be able to:
- calculate the mean rate of a reaction from given information about the quantity of a reactant used or the quantity of a product formed and the time taken
- draw, and interpret, graphs showing the quantity of product formed or quantity of reactant used up against time
- draw tangents to the curves on these graphs and use the slope of the tangent as a measure of the rate of reaction
- calculate the gradient of a tangent to the curve on these graphs as a measure of rate of reaction at a specific time.
#6.1.2
Factors which affect the rates of chemical reactions
Factors which affect the rates of chemical reactions include: the concentrations of reactants in solution, the pressure of reacting gases, the surface area of solid reactants, the temperature and the presence of catalysts.
Students should be able to recall how changing these factors affects the rate of chemical reactions.
#6.1.3
Collision theory and activation energy
Collision theory explains how various factors affect rates of reactions. According to this theory, chemical reactions can occur only when reacting particles collide with each other and with sufficient energy. The minimum amount of energy that particles must have to react is called the activation energy.
Increasing the concentration of reactants in solution, the pressure of reacting gases, and the surface area of solid reactants increases the frequency of collisions and so increases the rate of reaction.
Increasing the temperature increases the frequency of collisions and makes the collisions more energetic, and so increases the rate of reaction.
Students should be able to:
- predict and explain using collision theory the effects of changing conditions of concentration, pressure and temperature on the rate of a reaction
- predict and explain the effects of changes in the size of pieces of a reacting solid in terms of surface area to volume ratio
- use simple ideas about proportionality when using collision theory to explain the effect of a factor on the rate of a reaction.
#6.1.4
Catalysts
Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Enzymes act as catalysts in biological systems.
Catalysts increase the rate of reaction by providing a different pathway for the reaction that has a lower activation energy.
A reaction profile for a catalysed reaction can be drawn in the following form:
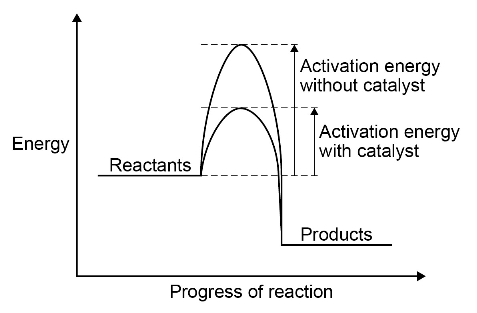
Students should be able to identify catalysts in reactions from their effect on the rate of reaction and because they are not included in the chemical equation for the reaction.
Students should be able to explain catalytic action in terms of activation energy.
Students do not need to know the names of catalysts other than those specified in the subject content.