#6.1.1a
the comparison of the Kekulé model of benzene with the subsequent delocalised models for benzene in terms of p-orbital overlap forming a delocalised π-system
Learners may represent the structure of benzene in equations and mechanisms as:
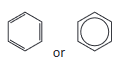
#6.1.1b
the experimental evidence for a delocalised, rather than Kekulé, model for benzene in terms of bond lengths, enthalpy change of hydrogenation and resistance to reaction (see also 6.1.1f)
#6.1.1c
use of IUPAC rules of nomenclature for systematically naming substituted aromatic compounds
Use of locant numbers to identify positions of substitution e.g. 2,4-dinitromethylbenzene.
#6.1.1d
the electrophilic substitution of aromatic compounds with:
(i) concentrated nitric acid in the presence of concentrated sulfuric acid
(ii) a halogen in the presence of a halogen carrier
(iii) a haloalkane or acyl chloride in the presence of a halogen carrier (Friedel–Crafts reaction) and its importance to synthesis by formation of a C–C bond to an aromatic ring (see also 6.2.4d)
Halogen carriers include iron, iron halides and aluminium halides.
#6.1.1e
the mechanism of electrophilic substitution in arenes for nitration and halogenation (see also 4.1.1h–i)
For nitration mechanism, learners should include equations for formation of NO2+.
Halogen carriers include iron, iron halides and aluminium halides.
For the halogenation mechanism, the electrophile can be assumed to be X+.
#6.1.1f
the explanation of the relative resistance to bromination of benzene, compared with alkenes, in terms of the delocalised electron density of the π-system in benzene compared with the localised electron density of the π-bond in alkenes (see also 4.1.3a, 6.1.1a)
#6.1.1g
the interpretation of unfamiliar electrophilic substitution reactions of aromatic compounds, including prediction of mechanisms
Extra information may be provided on exam papers.
#6.1.1h
the weak acidity of phenols shown by the neutralisation reaction with NaOH but absence of reaction with carbonates (see also 5.1.3b)
PAG7 (see also 6.3.1c)
#6.1.1i
the electrophilic substitution reactions of phenol:
(i) with bromine to form 2,4,6-tribromophenol
(ii) with dilute nitric acid to form a mixture of 2-nitrophenol and 4-nitrophenol
Note that nitration with phenol does not require concentrated HNO3 or the presence of a concentrated H2SO4 catalyst.
#6.1.1j
the relative ease of electrophilic substitution of phenol compared with benzene, in terms of electron pair donation to the π-system from an oxygen p-orbital in phenol (see also 4.1.3a)
Illustrated by reactions with bromine and with nitric acid.
Explanation is only in terms of susceptibility of ring to 'attack' and not in terms of stability of intermediate.
#6.1.1k
the 2- and 4-directing effect of electron-donating groups (OH, NH2) and the 3-directing effect of electron-withdrawing groups (NO2) in electrophilic substitution of aromatic compounds
Learners will not be expected to know further electron-donating or electron-withdrawing groups; relevant additional data will be supplied in examinations.
#6.1.1l
the prediction of substitution products of aromatic compounds by directing effects and the importance to organic synthesis (see also 6.2.5 Organic Synthesis).