#4.1.3a
alkenes as unsaturated hydrocarbons containing a C=C bond comprising a π-bond (sideways overlap of adjacent p-orbitals above and below the bonding C atoms) and a σ-bond (overlap of orbitals directly between the bonding atoms) (see also 4.1.2a); restricted rotation of the π-bond
Hybridisation is not required.
#4.1.3b
explanation of the trigonal planar shape and bond angle around each carbon in the C=C of alkenes in terms of electron pair repulsion (see also 2.2.2g–h, 4.1.2b)
#4.1.3c
(i) explanation of the terms:
- stereoisomers (compounds with the same structural formula but with a different arrangement in space)
- E/Zisomerism (an example of stereoisomerism, in terms of restricted rotation about a double bond and the requirement for two different groups to be attached to each carbon atom of the C=C group)
- cis–transisomerism (a special case of E/Z isomerism in which two of the substituent groups attached to each carbon atom of the C=C group are the same)
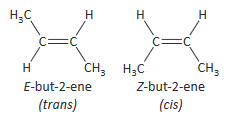
Use of E as equivalent to trans and Z as equivalent to cis is only consistently correct when there is an H on each carbon atom of the C=C bond.
(ii) use of Cahn–Ingold–Prelog (CIP) priority rules to identify the E and Z stereoisomers
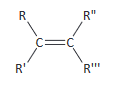
Assigning CIP priorities to double or triple bonds within R groups is not required:
#4.1.3d
determination of possible E/Z or cis–trans stereoisomers of an organic molecule, given its structural formula
#4.1.3e
the reactivity of alkenes in terms of the relatively low bond enthalpy of the π-bond
#4.1.3f
addition reactions of alkenes with:
(i) hydrogen in the presence of a suitable catalyst, e.g. Ni, to form alkanes
(ii) halogens to form dihaloalkanes, including the use of bromine to detect the presence of a double C=C bond as a test for unsaturation in a carbon chain
PAG7
(iii) hydrogen halides to form haloalkanes
(iv) steam in the presence of an acid catalyst, e.g. H3PO4, to form alcohols
#4.1.3g
definition and use of the term electrophile (an electron pair acceptor)
#4.1.3h
the mechanism of electrophilic addition in alkenes by heterolytic fission (see also 4.1.1h–i)
For the reaction with halogens, either a carbocation or a halonium ion intermediate is acceptable.
#4.1.3i
use of Markownikoff’s rule to predict formation of a major organic product in addition reactions of H–X to unsymmetrical alkenes, e.g. H–Br to propene, in terms of the relative stabilities of carbocation intermediates in the mechanism
Limited to stabilities of primary, secondary and tertiary carbocations.
Explanation for relative stabilities of carbocations not required.
#4.1.3j
addition polymerisation of alkenes and substituted alkenes, including:
(i) the repeat unit of an addition polymer deduced from a given monomer
(ii) identification of the monomer that would produce a given section of an addition polymer
#4.1.3k
the benefits for sustainability of processing waste polymers by:
(i) combustion for energy production
(ii) use as an organic feedstock for the production of plastics and other organic chemicals
(iii) removal of toxic waste products, e.g. removal of HCl formed during disposal by combustion of halogenated plastics (e.g. PVC)
#4.1.3l
the benefits to the environment of development of biodegradable and photodegradable polymers.