#4.1.1a
application of IUPAC rules of nomenclature for systematically naming organic compounds
Nomenclature will be limited to the functional groups within this specification.
E.g. CH3CH2CH(CH3)CH2OH has the systematic name: 2-methylbutan-1-ol.
Learners will be expected to know the names of the first ten members of the alkanes homologous series and their corresponding alkyl groups.
#4.1.1b
interpretation and use of the terms:
(i) general formula (the simplest algebraic formula of a member of a homologous series) e.g. for an alkane: CnH2n+2
(ii) structural formula (the minimal detail that shows the arrangement of atoms in a molecule) e.g. for butane: CH3CH2CH2CH3 or CH3(CH2)2CH3
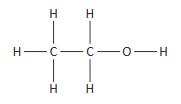
(iii) displayed formula (the relative positioning of atoms and the bonds between them) e.g. for ethanol:
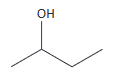
(iv) skeletal formula (the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional groups) e.g. for butan-2-ol:
See also 2.1.3b for empirical formula and molecular formula.
Definitions not required.
In structural formulae, the carboxyl group will be represented as COOH and the ester group as COO.
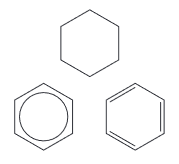
The symbols below will be used for cyclohexane and benzene:
#4.1.1c
interpretation and use of the terms:
(i) homologous series (a series of organic compounds having the same functional group but with each successive member differing by CH2)
(ii) functional group (a group of atoms responsible for the characteristic reactions of a compound)
(iii) alkyl group (of formula CnH2n+1)
(iv) aliphatic (a compound containing carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings)
(v) alicyclic (an aliphatic compound arranged in non-aromatic rings with or without side chains)
(vi) aromatic (a compound containing a benzene ring)
(vii) saturated (single carbon–carbon bonds only) and unsaturated (the presence of multiple carbon–carbon bonds, including C=C, C≡C and aromatic rings)
Definition required for homologous series only.
R may be used to represent alkyl groups, but also other fragments of organic compounds not involved in reactions.
The terms saturated and unsaturated will be used to indicate the presence of multiple carbon–carbon bonds as distinct from the wider term ‘degree of saturation’ used also for any multiple bonds and cyclic compounds.
#4.1.1d
use of the general formula of a homologous series to predict the formula of any member of the series
#4.1.1e
explanation of the term structural isomers (compounds with the same molecular formula but different structural formulae) and determination of possible structural formulae of an organic molecule, given its molecular formula
#4.1.1f
the different types of covalent bond fission:
(i) homolytic fission (in terms of each bonding atom receiving one electron from the bonded pair, forming two radicals)
(ii) heterolytic fission (in terms of one bonding atom receiving both electrons from the bonded pair)
#4.1.1g
the term radical (a species with an unpaired electron) and use of ‘dots’ to represent species that are radicals in mechanisms
Radical mechanisms will be represented by a sequence of equations.
Dots, •, are required in all instances where there is a single unpaired electron (e.g. Cl• and CH3•). Dots are not required for species that are diradicals (e.g. O).
#4.1.1h
a ‘curly arrow’ described as the movement of an electron pair, showing either heterolytic fission or formation of a covalent bond
‘Half curly arrows’ are not required, see 4.1.2f.
#4.1.1i
reaction mechanisms, using diagrams, to show clearly the movement of an electron pair with ‘curly arrows’ and relevant dipoles.
Any relevant dipoles should be included.
Curly arrows should start from a bond, a lone pair of electrons or a negative charge.