#4.44
know that an addition polymer is formed by joining up many small molecules called monomers
#4.45
understand how to draw the repeat unit of an addition polymer, including poly(ethene), poly(propene), poly(chloroethene) and (poly)tetrafluoroethene
#4.46
understand how to deduce the structure of a monomer from the repeat unit of an addition polymer and vice versa
#4.47
explain problems in the disposal of addition polymers, including:
- their inertness and inability to biodegrade
- the production of toxic gases when they are burned.
#4.48C
know that condensation polymerisation, in which a dicarboxylic acid reacts with a diol, produces a polyester and water
#4.49C
understand how to write the structural and displayed formula of a polyester, showing the repeat unit, given the formulae of the monomers from which it is formed including the reaction of ethanedioic acid and ethanediol:
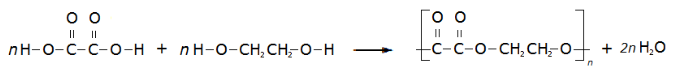
#4.50C
know that some polyesters, known as biopolyesters, are biodegradable