#11.2.1
First law of thermodynamics
Quantitative treatment of first law of thermodynamics, \(Q = ∆U + W \)
where Q is energy transferred to the system by heating, ∆U is increase in internal energy and W is work done by the system.
Applications of first law of thermodynamics.
#11.2.2
Non-flow processes
Isothermal, adiabatic, constant pressure and constant volume changes.
\(pV = nRT \)
adiabatic change: \(pV^γ = constant \)
isothermal change: \(pV = constant \)
at constant pressure \(W = pΔV \)
Application of first law of thermodynamics to the above processes.
#11.2.3
The p–V diagram
Representation of processes on p–V diagram.
Estimation of work done in terms of area below the graph.
Extension to cyclic processes: work done per cycle = area of loop
Expressions for work done are not required except for the constant pressure case, \(W = pΔV \)
#11.2.4
Engine cycles
Understanding of a four-stroke petrol engine cycle and a diesel engine cycle, and of the corresponding indicator diagrams.
Comparison with the theoretical diagrams for these cycles; use of indicator diagrams for predicting and measuring power and efficiency
input power = calorific value × fuel flow rate
Indicated power as (area of p−V loop) × (no. of cycles per second) × (no. of cylinders)
Output or brake power, \(P = Tω \)
friction power = indicated power – brake power
Engine efficiency; overall, thermal and mechanical efficiencies.
\(\text{Overall efficiency} = \dfrac{\text{brake power}}{\text{input power}} \)
\(\text{Thermal efficiency} = \dfrac{\text{indicated power}}{\text{input power}} \)
\(\text{Mechanical efficiency} = \dfrac{\text{brake power}}{\text{indicated power}} \)
A knowledge of engine constructional details is not required.
Questions may be set on other cycles, but they will be interpretative and all essential information will be given.
#11.2.5
Second Law and engines
Impossibility of an engine working only by the First Law.
Second Law of Thermodynamics expressed as the need for a heat engine to operate between a source and a sink.
efficiency \(= \dfrac{W}{Q_H} = \dfrac{Q_H - Q_C}{Q_H} \)
maximum theoretical efficiency \(= \dfrac{T_H - T_C}{T_H} \)
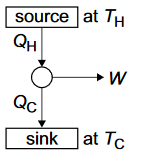
Reasons for the lower efficiencies of practical engines.
Maximising use of W and QH for example in combined heat and power schemes.
#11.2.6
Reversed heat engines
Basic principles and uses of heat pumps and refrigerators.
A knowledge of practical heat pumps or refrigerator cycles and devices is not required.
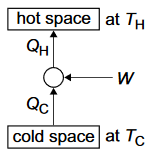
Coefficients of performance:
refrigerator: \(COP_{ref} = \dfrac{Q_C}{W} = \dfrac{Q_C}{Q_H-Q_C} = \dfrac{T_C}{T_H-T_C} \)
heat pump: \(COP_{hp} = \dfrac{Q_H}{W} = \dfrac{Q_H}{Q_H-Q_C} = \dfrac{T_H}{T_H-T_C} \)